The inception of ANeuroTech is the result of my +3 decades of experience in the clinical development of neuroscience blockbusters in collaboration with Big Pharma.
My target is to bring an effective treatment for people that continue to suffer from depression with associated subjective cognitive problems.
Dr. Erik Buntinx, Psychiatrist, Inventor & Founding CEO of ANeuroTech
Treating Partially Responsive Depression (PRD) as an unmet medical need
ANEUROTECH is developing its late stage Phase III drug ANT01 in Partial Responsive Depression (PRD). ANT01 is an once daily, orally-administered small molecule to attack Subjective Cognitive Decline and Potentiate Initial Antidepressant Therapies.
This established drug acts uniquely as a combined High Selective Serotonin 2A / Dopamine 4 receptor (5-HT2A/D4DR) Blocker in the brain.
ANeuroTech in the media
Partial Responsive Depression (PRD): a significant unmet medical need.
In the US, there are only 4 drugs approved for the adjunctive treatment of Partial Depressive Disorder (PRD), i.e., quetiapine, aripiprazole, brexpiprazole and cariprazine.
Unfortunately, these atypical antipsychotics come with a significant side‑effect burden. These include most frequently the risk of various motor side effects and the metabolic syndrome and are responsible for a significant problem of non-adherence and moreover non durability of treatment.
However, PRD represents more than 60% of the initial AD treated patients having an annual incidence of around 1.8%!
As such PRD represents a significant unmet medical need.
This has been demonstrated in STARD (Sequenced Treatment Alternatives to Relieve Depression) , the largest randomized trial of the treatment of major depressive disorder (MDD) where only 36.8% of a representative sample of outpatients with MDD achieved remission after a first course of antidepressant treatment.
Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163:1905–17.
** Ferrari AJ, et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature, Psychological Medicine , Volume 43 , Issue 3 , March 2013 , pp. 471 – 481.
Adjunctive Treatment of Depression with ANT01
An unique late stage opportunity.
Based on our in-dept knowledge on involved brain mechanisms in depression, we believe that in contrast to the mechanism of action of current first line antidepressants such as the selective serotonergic and noradrenergic re-uptake inhibitors (SSRIs/SNRIs), a highly selective 5-HT2A/D4 antagonist such as ANT01, is able to improve the Innate Emotional and Cognitive Brain Function.
In particular, we believe that key symptoms such as emotional blunting and cognitive impairment which are present as part of the pathophysiology of the target patients i.e., patients suffering from MDD, and which typically are not well responding to SSRIs/SNRIs, might be improved by adding ANT01.
Since any drug in the art is known with ANT01’ Mode of Action, our late stage development program is potentially offering a unique opportunity in treating PRD.

Market Opportunities
Taken in to account that only in the US around 3 M patients per year are diagnosed with PRD after an initial treatment with an antidepressant, a superior add-on treatment option as with ANT01, represents a major market opportunity.
As a base case, with a similar pricing as virtioxetine and a mean 6 month treatment in only 10% of newly diagnosed target patients, ANT01 could hit an annual sales of over $1.5b. However, with a mean 9/12 month treatment we assume treatment in 15/20% of newly diagnosed target patients and an annual peak sales of USD 3.3 to 6.0 billion respectively.
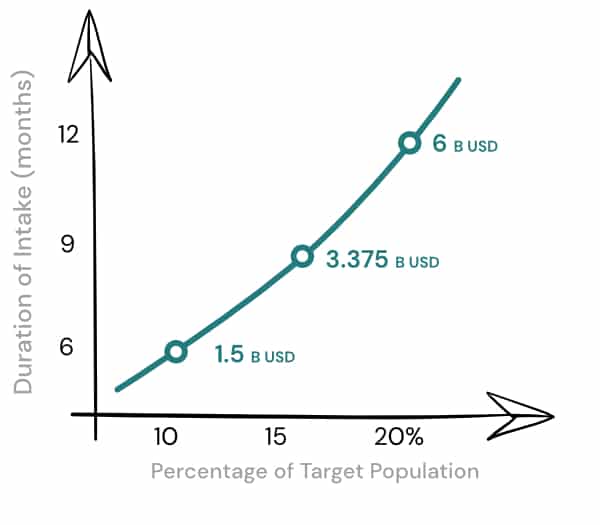
Percentage of Target Population increases in relation to Duration of Intake since Total Target Population is limited.